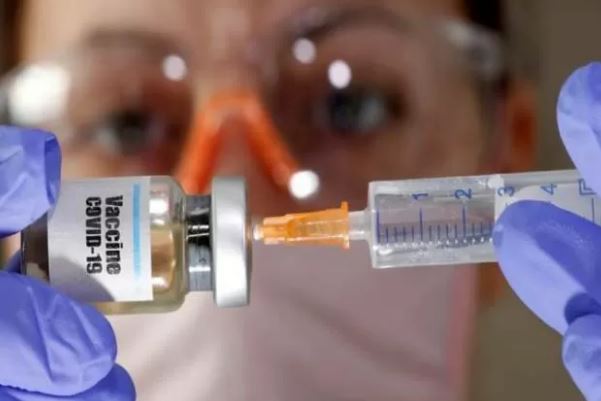
Serum Institute Permits Emergency Use of ‘Covishield’, Becomes First Indian Company
Indian vaccine manufacturer Serum Institute of India (SII) has sought formal approval for the emergency use of “Covishield”, a Covid-19 vaccine from Oxford in India on 06 December 2020. According to media reports, Serum has sought permission from the Controller General of Indian Medicine (DCGI) to use the emergency of its Corona vaccine ‘Kovishield’. With this, the Serum Institute of India has become the first indigenous company in the country. Earlier, on 05 December 2020, the Indian unit of American drug maker Pfizer sought formal approval for the emergency use of the Covid-19 vaccine developed by it. Prior to this,

 Login/Register
Login/Register